Select Region / Language
Recently, Snibe has passed the IFCC External Quality Assessment Scheme for Reference Laboratories in Laboratory Medicine (IFCC-RELA), and 9 assays from Snibe reference laboratory achieved excellent results during the assessment, including 25-OH-Vitamin D3, 17-OH Progesterone, AP, ALT, Amylase, AST, CK, GGT and LDH.

IFCC-RELA is organized on behalf of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) by the Reference Institute of Bioanalysis (RfB) of the German Society of Clinical Chemistry and Laboratory Medicine (DGKL). It represents the highest measurement level of clinical chemistry and laboratory medicine, and is recognized as one of the most important quality evaluation activities in the field of international clinical chemistry and laboratory medicine.
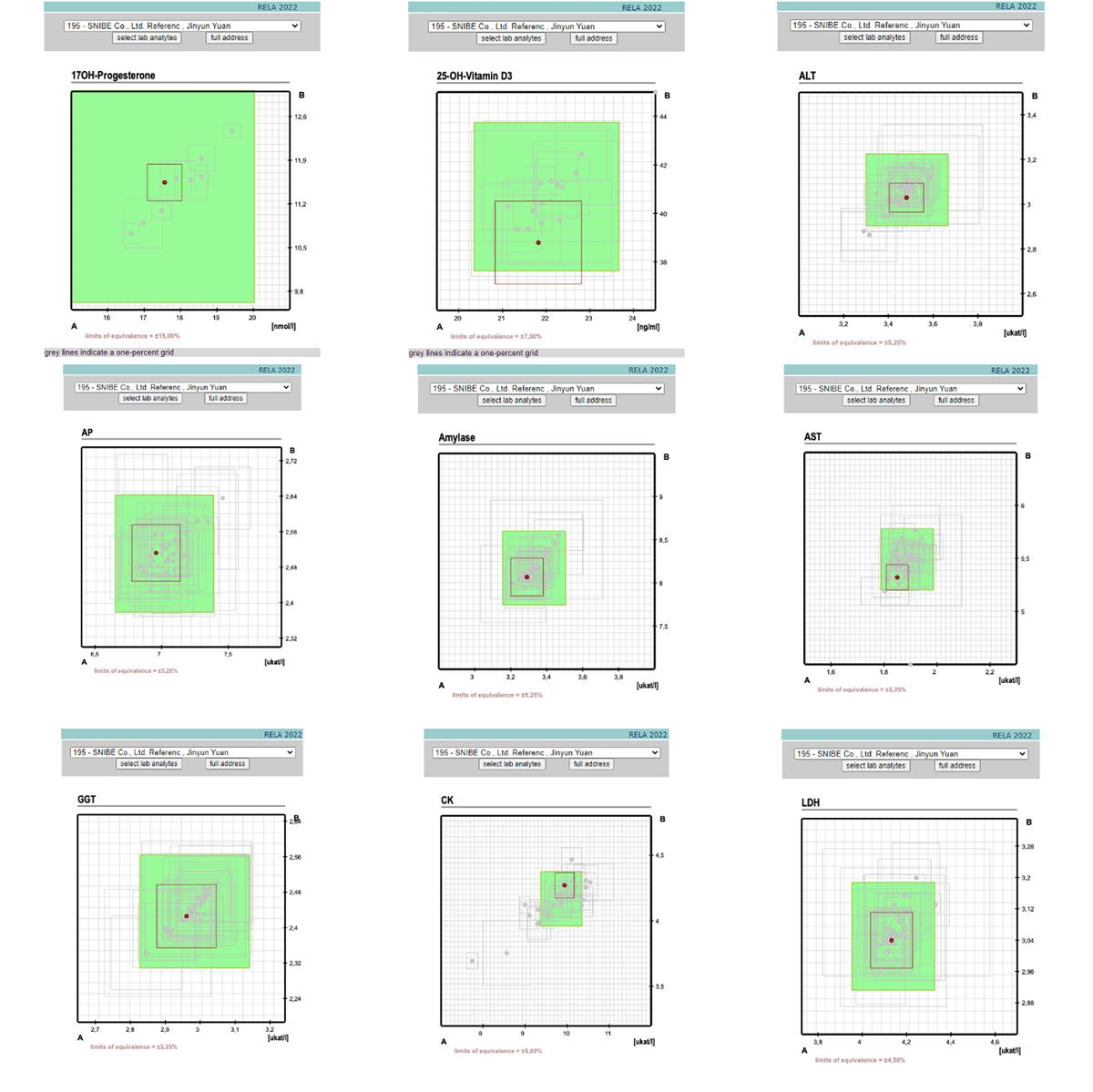
Results for 195-SNIBE Co., Ltd. Reference Measurement Laboratory
Snibe reference laboratory has two main platforms at present, namely, Ultraviolet-visible Spectrophotometer and an ultra-high-performance Liquid Chromatography-tandem Mass Spectrometer, which effectively guarantee the traceability and standardization of the test results of Snibe products. Through the IFCC-RELA, Snibe proved its technical ability of laboratory reference measurement can reach the highest measurement level of clinical chemistry and laboratory medicine.

In the future, Snibe will continue to strengthen its quality of products, ensure that clinical measurement results can be traced to international standards, build an uninterrupted traceability chain of "International System of Units (SI)—Reference Laboratory—Clinical Laboratory", so as to ensure the accuracy of measurement results, and achieve mutual recognition of clinical measurement results.
[1] Accesse at:http://dgkl-rfb.de:81/



