 |
A Monthly Snibe Newsletter
|
|
|

|
|
|
|
|
Identify Respiratory Infection, Prevent Disease Outbreak
|
|
|
|
|
|
|
|
|
|
The Recommended Methods for Respiratory Infection Diagnosis
|
|
The Expert Consensus on Clinical Diagnosis and Treatment of Respiratory Infections During Epidemics of Multiple Pathogens highlights the IgM and IgG antibody testing because of its high sensitivity and specificity.
The consensus also emphasizes that recent IgM seroconversion or a fourfold increase in IgG titer serves as an effective supplementary diagnostic criterion [1, 6].
|
|
|
|
|
|
|
Snibe Now Offering Comprehensive Respiratory Pathogens Diagnostics
|
|
Snibe has recently obtained seven new registration certificates for respiratory infection diagnosis, with these additions, Snibe now holds a total of 14 registration certificates, covering diagnostics for 10 major respiratory pathogens.
|
|
|
|
|
|
|
|
Snibe Comprehensive Respiratory Infectious Diagnosis Solution
|
Symptoms caused by different pathogens are often similar in the early stages of upper respiratory tract infections, manifesting as mild signs such as a runny nose, nasal congestion, sneezing, and throat discomfort, with or without fever.
Although symptoms of respiratory infections often overlap, treatment approaches vary significantly [4]. Co-infections further complicate diagnosis and management, making timely detection of causative pathogens essential for precise and effective treatment.
Snibe now offers 14 comprehensive testing options, including influenza A and B, mycoplasma, chlamydia, SARS, respiratory syncytial virus, coxsackievirus, huamn parainfluenza, legionella pneumophila and adenovirus, to help clinicians differentiate diagnoses and provide appropriate treatment plans.
|
|
|
Mycoplasma pneumoniae IgG
Mycoplasma pneumoniae IgM
Chlamydia pneumoniae IgG
Chlamydia pneumoniae IgM
2019-nCoV IgG
2019-nCoV IgM
SARS-CoV-2 Ag
SARS-CoV-2 S-RBD IgG
SARS-CoV-2 Neutralizing Antibody
|
|
|
|
*Influenza A Virus IgM
*Influenza B Virus IgM
*Human Parainfluenza Virus IgM
*Legionella pneumophlia IgM
- Respiratory Syncytial Virus:
*Respiratory Syncytial Virus IgM
*Coxsackievirus B IgM
*Adenovirus IgM
|
|
|
|
|
|
|
Global Main Types of Respiratory Infections[3, 4, 5]
|
|
|
|
|
Globally, respiratory infections remain a significant health burden. Lower respiratory infections, including pneumonia, are among the leading causes of mortality in children under five, responsible for over 808,000 deaths in 2017—representing 15% of all deaths in this age group. Adults over 65 and those with pre-existing health conditions are also at high risk.
Viral and bacterial pathogens such as influenza viruses, respiratory syncytial virus, and Streptococcus pneumoniae are the most prevalent causes, often leading to severe complications and even mortality without timely diagnosis and treatment.
|
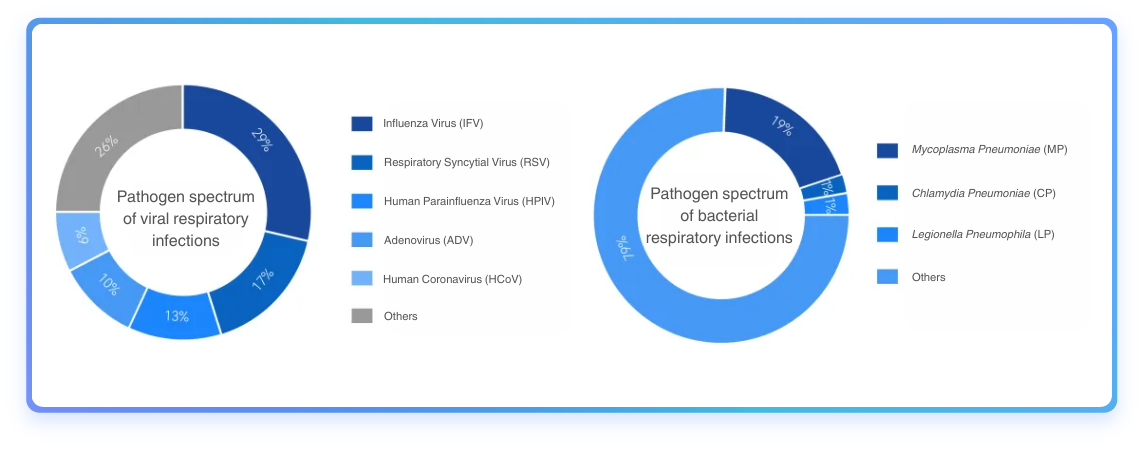
Fig1. Respiratory infection pathogen spectrum. [1, 2]
|
|
|
Acute Respiratory Infections in India [7, 8]
|
|
|
The seasonal incidence of respiratory viral infections in India shows a clear pattern, with peaks typically occurring during the winter months. Influenza A, particularly the H1N1 strain, has been observed to peak during the monsoon season, but cases often continue into the winter, leading to a dual burden of respiratory infections.
The winter season is associated with increased hospitalizations due to acute respiratory diseases (mostly RSV), particularly among pediatric populations, as evidenced by data from recent years.
|
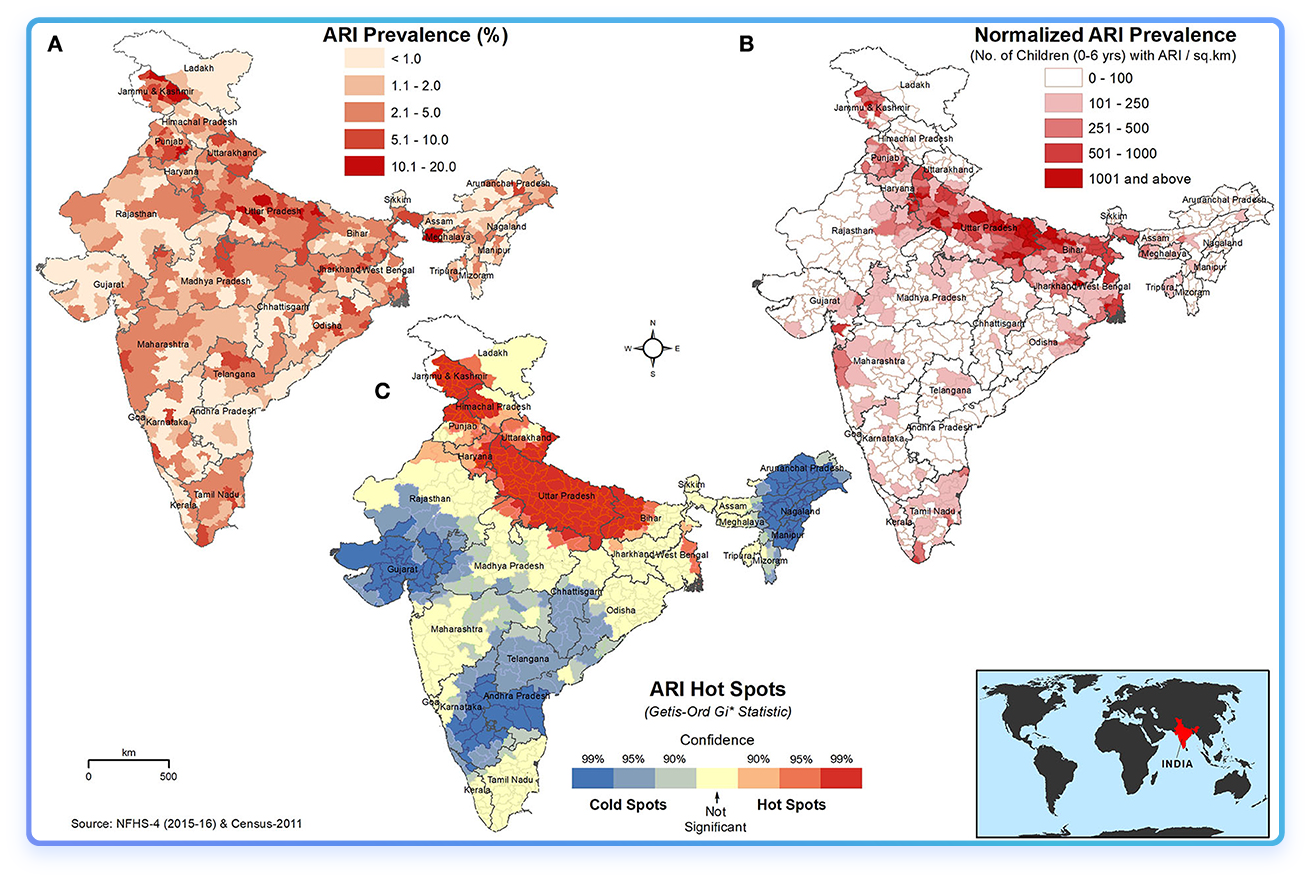
Fig2. Spatial distribution of (A) absolute percentage of ARI in children under 5 years, (B) ARI prevalence normalized to the children population density (children with ARI/km2), and (C) hot and cold spots of ARI in children under 5 years. The darker red shades indicate higher values/hotspots of ARI. The inset map shows the location of India on the world map. [9]
|
|
|
[1] Gao, X., Luo, Y., et al. (2024). Expert Consensus on Diagnosis and Treatment of Acute Respiratory Infections in Adult Outpatients and Emergency Departments. Chinese Journal of Infectious Diseases, 42(00), E01–E29.
[2] Li, Z.-J., et al. (2021). Etiological and epidemiological features of acute respiratory infections in China. Nature Communications, 12(1), 5026.
[3] World Health Organization. (2018). Pneumonia fact sheet. Retrieved from [https://www.who.int] (https://www.who.int).
[4] GBD 2017 Causes of Death Collaborators. (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1736-1788.
[5] Collaborators of the Global Burden of Disease Study 2024. (2024). Number of deaths from pneumonia in children under five. Retrieved November 19, 2024, from [Our World in Data] (https://ourworldindata.org/grapher/number-of-deaths-from-pneumonia-in-children-under-5).
[6] National Health Commission of the People's Republic of China, National Administration of Traditional Chinese Medicine. (2019). Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Children (Issue 01, 002, 1674-2397).
[7] Anusha Hindupur, Thangam Menon, Prabu Dhandapani, Epidemiology of respiratory syncytial virus infections in Chennai, south India, Clinical Epidemiology and Global Health, Volume 7, Issue 3, 2019, Pages 288-292, ISSN 2213-3984,
[8] Rupa V, Isaac R, Manoharan A, Jalagandeeswaran R, Thenmozhi M. Risk factors for upper respiratory infection in the first year of life in a birth cohort. Int J Pediatr Otorhinolaryngol. 2012 Dec;76(12):1835-9. doi: 10.1016/j.ijporl.2012.09.013. Epub 2012 Sep 29. PMID: 23031180.
[9] Balasubramani K, Prasad KA, Kodali NK, Abdul Rasheed NK, Chellappan S, Sarma DK, Kumar M, Dixit R, James MM, Behera SK, Shekhar S and Balabaskaran Nina P (2022) Spatial epidemiology of acute respiratory infections in children under 5 years and associated risk factors in India: District-level analysis of health, household, and environmental datasets. Front. Public Health 10:906248. doi: 10.3389/fpubh.2022.906248
|
|
|
|
|
|
Should you find our products or information intriguing, please do not hesitate to establish contact with us
For further details, kindly click the button below to send an email to sales@snibe.com
We will reach out to you via email and offer our assistance
|
| |
|
|
|
|
|
|
|
|



